In clinical development, every day matters.
When we talk about time, we are not only talking about expenses but also revenue.
Even a single day of delay can result in hundreds of thousands, sometimes millions, in lost revenue. For therapies with blockbuster potential, each month of delay can mean tens or even hundreds of millions in unrecoverable losses. In the case of a drug like Keytruda, which generates $8billion in annual sales, the financial impact of delays becomes truly staggering.
For biotechs, these delays may determine whether the next financing round is secured – potentially impacting company survival. For large pharma, delays shorten the period of market exclusivity before generics enter and weaken the pipeline. In both cases, the result is the same: time lostis the revenue lost.
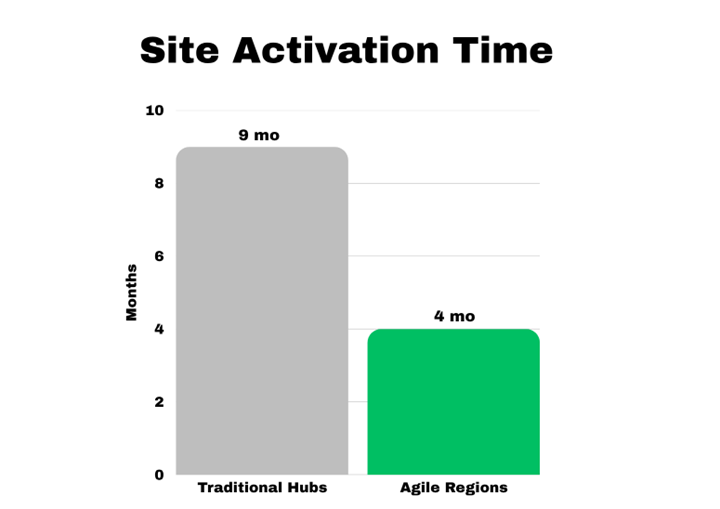
Much of the delay is avoidable. On average, sites lose two and more months of recruitment time due to delays in the study start-up – a common andcostly issue. Seemingly small pauses one by one accumulate into a long stretch of general study timelines.
Site activation time significantly varies across the regions.
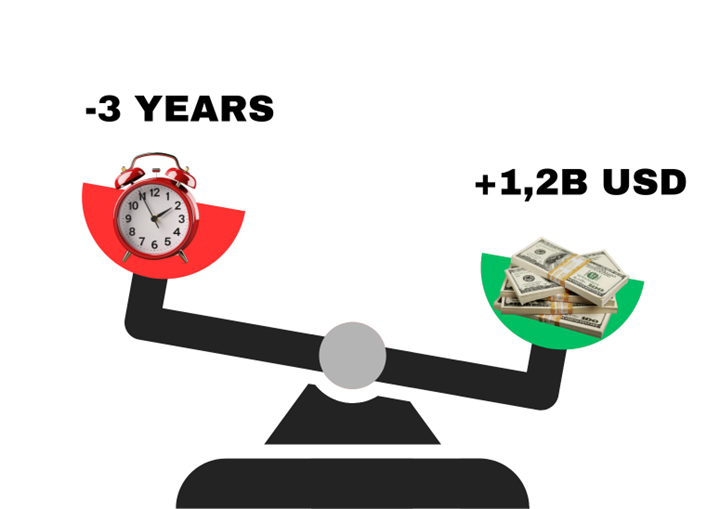
Consider a rare disease program projected to generate 400M dollars annually. On the conventional ten-year path from discovery to approval, revenue does not begin until year ten, meaning a full decade of investment without return while patients continue to wait.
Now imagine that streamlined contracting, accelerated regulatory pathways, and global enrollment strategies reduce that time to seven years. The company gains three full years of earlier market presence.
The impact of this acceleration is extraordinary. It represents not simply costsavings but 1.2 billion dollars in revenue captured sooner, enabling faster patient access, boosting investor confidence, and securing stronger competitive position. Those three years can determine whether a company leads its therapeutic space or is overtaken by rivals who moved more quickly.
Timelines must be viewed as revenue strategy – not as administrative overhead. Leaders who prioritize speed in contracting, regulatory submissions, and site activation don’t just improve efficiency; they actively shift the revenue curve forward. This means delivering therapies to patients sooner, demonstrating real-world impact to investors, and strengthening the company’s competitive edge.
At People Value Research, we see this reality every day. Through rapid approvals, motivated investigators, and access to underutilized patient populations across our geographical coverage we help sponsors compress timelines and bring revenue forward.
For biotech and pharmaceutical companies, the key question is no longer just “What does this trial cost?” – it must also be “How much revenue is being lost by moving slowly?”
In clinical development, the true cost is not what you spend. It is what you fail to earn while the clock keeps ticking ⏰